Mass Tort Product Liability and Its Potential Intersection with Pharmaceutical Liability under the False Claims Act
Table of Contents
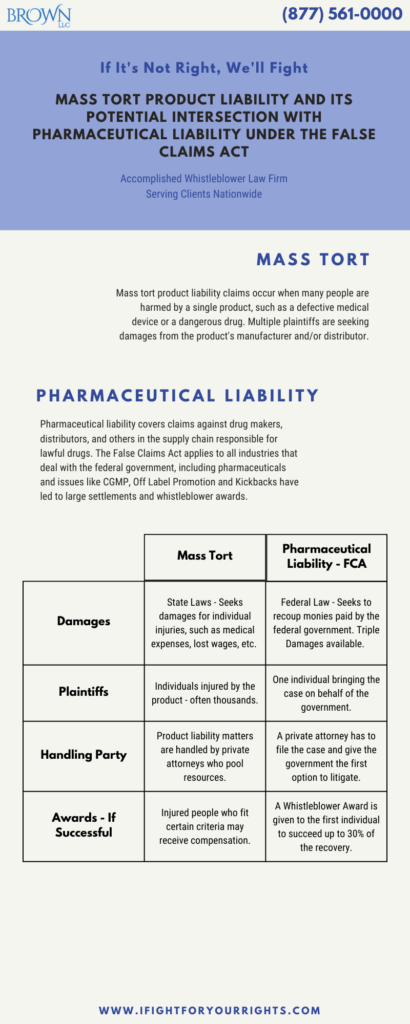
Mass tort product liability claims arise when a large number of people are harmed or injured by the same product, such as a defective medical device or a dangerous drug. These cases are often complex and involve multiple plaintiffs seeking damages from the same defendant or defendants who were involved in the manufacturer or distribution of the product. In 2023, the biggest mass torts that are moving forward involve Camp Lejeune water contamination, hair relaxer cancer link, the hopeful conclusion of the talcum powder – ovarian cancer litigation, and the Zantac – cancer link which unfortunately, all of the federal cases were dismissed and will be on appeal as the state cases surge forward. Zantac is an example of potential pharmaceutical liability.
Pharmaceutical liability involves claims against drug manufacturers, distributors, and others in the supply chain who are responsible for the development, manufacture, and distribution of lawful drugs. In some cases, these two areas of law can intersect, particularly in cases involving the False Claims Act (FCA). The False Claims Act is a federal law that imposes liability on individuals and companies that defraud the government by submitting false claims for payment. The FCA applies to all industries that conduct business with the federal government, including the pharmaceutical industry. In recent years, there has been a growing trend of using the FCA to pursue claims against drug manufacturers and others in the pharmaceutical supply chain.
Product liability negligence refers to the legal responsibility of a manufacturer, distributor, or seller for injuries or damages caused by a defective product, and understanding the concept of product liability negligence is crucial in navigating the complexities of product liability cases.
The intersection of mass tort product liability and pharmaceutical product liability under the FCA can be complex, but there are several key issues that are worth exploring in more detail. For an umbrella issue, commencing a product liability mass tort involves each individual having particularized damages from the product at issue, in contrast, under the FCA, the taxpayers are the victim and if successful, there is one recovery for the government and a potential whistleblower award. In contrast, the mass tort, thousands of people may receive compensation. Product liability matters are handled by private attorneys who often pool resources to create a steering committee and litigation fund as the costs for litigation are significant, as opposed to a FCA where the true party is the government and they have the first crack at the case, and only the first to file under the FCA has the rights to pursue the action. A mass tort is not a class action, although if there is a mass tort settlement, certain elements will resemble a class, whereas a False Claims Act settlement just resolves the claims regarding the fraud against the government and the whistleblowers stake in the claim.
It is important to understand the difference between the types of damages that can be recovered in mass tort product liability cases and FCA cases. In a mass tort product liability case, plaintiffs seek damages for their injuries, such as medical expenses, lost wages, and pain and suffering. In a pharmaceutical product liability under the FCA case, the government seeks damages for the harm caused by the defendant’s fraudulent conduct, such as the amount of money that was paid out as a result of the false claims. FCA damages are often much higher than the damages sought in an individual mass tort product liability case, however, the aggregate mass tort settlement often exceeds the FCA settlement, since the aggregate is often for tens of thousands of plaintiffs.
It is worth considering the types of conduct that can give rise to liability under the FCA. The FCA imposes liability on individuals and companies who knowingly submit false claims for payment to the government. This can include claims for payment for drugs that were not approved by the FDA, payment for pharmaceutical products that were adulterated or falsely passed inspection, claims for payment for off-label uses of drugs, and claims for payment for drugs that were not manufactured in accordance with FDA regulations. Also, kickbacks are strictly prohibited, so providing something of value to induce prescriptions have led to major FCA settlements and judgments.
Speak with the Lawyers at Brown, LLC Today!
Over 100 million in judgments and settlements trials in state and federal courts. We fight for maximum damage and results.
It is important to understand the role that whistleblowers can play in pharmaceutical product liability cases. The FCA allows individuals to bring claims on behalf of the government, known as qui tam claims. Whistleblowers who bring qui tam claims are entitled to a percentage of any damages recovered by the government as a whistleblower reward. In recent years, there has been a surge in qui tam claims brought by individuals who have inside knowledge of fraudulent conduct by drug manufacturers and others in the pharmaceutical supply chain which has resulted in billions of dollars of recoveries and hundreds of millions of dollars in whistleblower awards.
The role of the whistleblower may be huge in both types of cases. In the mass tort the insider may have information that damns the company, establishes liability and allows the plaintiffs to make their proofs opening the door to significant damages. However, the mass tort whistleblower per se isn’t entitled to any money for their information! Only, if the whistleblower files a qui tam asserting that due to the malady with the product, had the government known about its insufficiency it wouldn’t have paid for it. Then if the government seeks to recoup its payments, the whistleblower can receive up to 30% of what the government recovers.
There have been several successful cases where mass tort product liability claims and FCA claims have intersected. For example, in 2012, GlaxoSmithKline paid $3 billion to settle claims that it had marketed certain drugs for off-label uses and had failed to report safety data to the FDA. This settlement included both FCA claims and claims brought by plaintiffs in mass tort product liability cases. In another case, Johnson & Johnson paid 2 billion to settle claims that it had marketed certain drugs for off-label uses and had paid kickbacks to physicians and pharmacists. This settlement also included both FCA claims and claims brought by plaintiffs in mass tort product liability cases.
The intersection of mass tort product liability and pharmaceutical liability under the False Claims Act can be complex, but it can also provide an opportunity for plaintiffs to recover damages for their injuries and for the government to hold drug manufacturers and others in the pharmaceutical supply chain accountable for their fraudulent conduct. If you have been injured by a dangerous drug or medical device, or if you have inside knowledge of fraudulent conduct by a drug manufacturer or other company in the pharmaceutical supply chain, it is important to speak with an experienced whistleblower attorney who can help you understand your rights and explore your legal options.