6 Biggest False Claims Act Healthcare Fraud Cases of 2024 + One Defense Contractor Fraud FCA

Table of Contents
Last year, 2024, marked yet another record-breaking year for False Claims Act (FCA) settlements with the Department of Justice (DOJ) resulting in a record amount of cases filed and resolving more than $1 billion in FCA settlements within the first half of the year alone. (1)
There have been significant strides in healthcare anti-fraud efforts since the first half of the year with $2.9 billion being recovered by the DOJ in Fiscal Year 2024. Of the $2.9 billion recovered, over $1.67 billion was recovered from alleged fraud affecting the healthcare industry.(2) There has been an increase in alleged healthcare fraud cases, which compose some of the largest FCA settlements to date.(2)
Many, if not all the cases that settled are due to the persistent efforts of whistleblowers and their whistleblower lawyers. These efforts have included combating the opioid epidemic, exposing healthcare organizations that bill for unnecessary services and substandard care, exposing healthcare fraud schemes involving unlawful kickbacks as well as Stark Law violations; thus, there are many forms of alleged healthcare fraud from which the government has recovered.(2)
In celebration of yet another successful year in exposing healthcare fraud, here are six of the largest healthcare fraud cases of 2024.
$2.75 Billion- 193 charged in DOJ’s 2024 National Health Care Fraud Enforcement Action
Since March 2007, when the Health Care Fraud Unit was created as a branch of the Department of Justice (DOJ), it has been dedicated to combating healthcare fraud across the United States with prosecutors being stationed all over the country to provide greater contributions to the continuous fight against healthcare fraud. The Health Care Fraud Unit has been successful in charging over 5,400 defendants for fraudulently billing Medicare, Medicaid, and other private insurers since the department’s inception. With a constant rise in reporting of healthcare fraud since 2007, coupled with rising skepticism towards the healthcare sphere, 2024 has proven to be a historic year for healthcare fraud whistleblowers.(3)
On Thursday, June 27, 2024, the Justice Department announced an action which led to criminal charges being placed against 193 defendants. Of those 193 defendants, 76 were doctors, nurse practitioners, and other licensed medical professionals in 32 federal districts.(3)
The recovery of $2.75 billion covers various forms of alleged healthcare fraud:
- $900 million recovered for an alleged scheme related to amniotic wound grafts
- $90 million recovered for fraud allegedly committed by corporate executives that distributed mislabeled and adulterated HIV medications
- $146 million recovered in relation to alleged fraudulent addiction treatment schemes
- Over $450 million in other alleegd healthcare fraud and opioid schemes
Find additional details about the National Health Care Fraud Enforcement Action here.
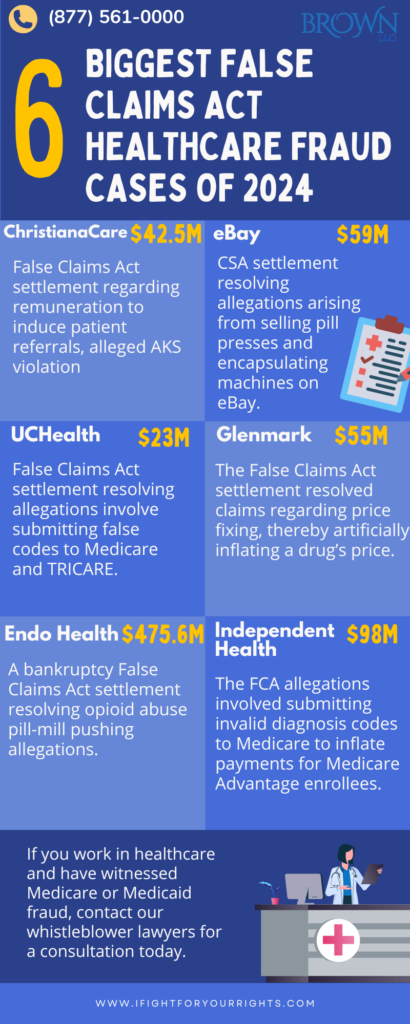
ChristianaCare Settlement
In one of the first healthcare fraud cases to settle in 2024, ChristianaCare paid $42.5 million to resolve allegations of healthcare fraud brought under the False Claims Act (FCA) on January 4, 2024. The case originated from a whistleblower complaint filed by the organization’s former chief compliance officer that alleged ChristianaCare provided illegal remuneration to non-employee neonatologists and surgeons through ancillary support services to induce patient referrals to its hospitals. This alleged arrangement violated the Anti-Kickback Statute and the Stark Law, which are designed to prevent medical decisions from being influenced by provider profit rather than patient care.(4)
The government contended that these practices led to the submission of false claims to Medicare and Medicaid. The settlement amount is $42.5 million, which is allocated between the United States and the State of Delaware based on the value of the underlying healthcare claims.
Find additional details about ChristianaCare’s settlement here.
eBay Settlement
This past year also marked a significant marker in the history of alleged healthcare fraud as the first Controlled Substances Act settlement with an E-Commerce company occurred in the first month of 2024.
On January 31st, 2024, eBay Inc., the e-commerce company, agreed to pay $59 to resolve alleged violations of the Controlled Substances Act (CSA). The allegations stem from the sale of pill presses and encapsulating machines through eBay’s website.(5)
These machines can be used to manufacture illegal drugs such as counterfeit pills, and are also subject to CSA regulations, which include requirements for identity verification of purchasers, record-keeping, and reporting to the Drug Enforcement Administration.
The investigation revealed that numerous eBay pill press buyers also purchased counterfeit molds and dies, and many of these buyers were subsequently prosecuted for drug-related crimes. The settlement requires eBay to pay $59 million and enhance its compliance program regarding the sale of restricted items. This settlement is noteworthy as the fourth-largest of its kind and as the first with an e-commerce company, highlighting the Department of Justice’s commitment to ensuring compliance with the CSA by entities involved in the sale of equipment used to make counterfeit pills.(5)
UCHealth Settlement
On November 12, 2024, University of Colorado Health (UCHealth) agreed to pay $23 million to resolve allegations of fraudulent billing practices for emergency department visits. The allegations pertain to the false coding of certain Evaluation & Management (E&M) claims submitted to Medicare and TRICARE.(6)
Specifically, the United States argued that UCHealth automatically used the highest resource usage code (CPT 99285) when a patient’s vital signs were checked more times than the hours spent in the emergency room, regardless of the severity of the patient’s condition or the resources used, unless the patient was in the ER for less than 60 minutes.
The settlement also includes a payment of $3.91 million to the whistleblower who brought the case under the False Claims Act as a whistleblower award.(6)
Speak with the Lawyers at Brown, LLC Today!
Over 100 million in judgments and settlements trials in state and federal courts. We fight for maximum damage and results.
Glenmark Settlement
On September 4, 2024, Glenmark Pharmaceuticals Inc., a generic drug manufacturer, agreed to a $55 million settlement to resolve allegations of price-fixing. The settlement includes a $25 million civil False Claims Act settlement and an additional $30 million for criminal consequences.(7)
The lawsuit alleges that Glenmark both received and paid out compensation through arrangements on the drug Pravastatin’s price, supply, and distribution with other pharmaceutical manufacturers between 2013 and 2015 in an attempt to ‘fix’ its price.(8) These alleged actions are a violation of the Anti-Kickback Statute, a federal law that prohibits offering, paying, soliciting, or receiving anything of value to induce or reward referrals or business on products or services covered by federal healthcare programs.
The whistleblower who reported this fraud to the government may receive a whistleblower reward in the $6 million range. The False Claims Act also protects whistleblowers from retaliation, which only encourages more individuals to report unethical practices in the healthcare industry.
For additional details regarding the settlement, read the firm’s article on the matter here.
Endo Health Settlement
On February 29, 2024, Endo Health Solutions Inc. (EHSI) reached a global resolution with the U.S. Justice Department regarding criminal and civil investigations into the sales and marketing of their opioid drug, Opana ER. (9) The charges stem from EHSI’s marketing practices from 2011 to 2017, which included misrepresenting Opana ER’s safety by falsely claiming it was tamper- and crush-resistant despite lacking the clinical data to support such a claim. The company also allegedly targeted healthcare providers known to prescribe high levels of opioids, sometimes ignoring internal concerns about potential abuse.(9)
This resolution also includes a civil settlement where EHSI will pay $475.6 million to resolve its liability under the False Claims Act (FCA) for losses to federal healthcare programs.(9) In addition, a bankruptcy settlement was reached where the government will be paid up to $464.9 million over 10 years. The resolution also requires EHSI to cease operations in its current form and not emerge from bankruptcy.
As part of the settlement, EHSI must make millions of documents related to its role in the opioid crisis public. (9) The reorganized company must also fund trusts for opioid-related claims and pay over $450 million to state, municipal, and tribal entities to support addiction treatment programs. The company also had to pay an additional $200 million when it emerged from bankruptcy back in April, as well as up to an additional $100 million over five years if certain financial goals are met.
Independent Health Settlement
On December 20, 2024, Independent Health, a Medicare Advantage provider, agreed to pay up to $98 million, including $34.5 million in guaranteed payments and up to $63.5 million in contingent payments to resolve False Claims Act allegations placed against it. The allegations center around the submission of invalid diagnosis codes to Medicare to inflate payments for Medicare Advantage Plan enrollees.(10)
It is alleged that Independent Health utilized a subsidiary, DxID LLC, to search medical records and solicit physicians for information to support services that would result in increased payments.(10) The government contends that from 2011 to 2017, Independent Health knowingly submitted unsupported diagnoses to the Centers for Medicare and Medicaid Services (CMS) to increase payments.
The settlement also incorporates a five-year corporate integrity agreement (CIA), which requires Independent Health to hire an independent review organization. The whistleblower in this matter will receive at least $8,212,500 of the settlement.
Raytheon: Significant Strides in FCA Litigation
With all of the success the Department of Justice has had in recovering fraudulent funds in the healthcare industry, it is important to note the significant strides made in other fields of FCA litigation. For instance, whistleblower law firm Brown, LLC played a crucial role in the Raytheon settlement, successfully representing a whistleblower who exposed fraudulent pricing and foreign bribery. The firm’s efforts led to a $950 million settlement in October 2024, further solidifying its reputation as a leader in whistleblower litigation.(11)
Brown, LLC emphasizes the importance of public-private cooperation in fighting corporate fraud and ensuring that those who expose wrongdoing are protected and rewarded.(11)
These cases, among the countless other settlements that have led to billions of dollars being recovered by the government, serve as a warning to other contractors that defrauding the government is illegal and will be punished, while also showing how effective a whistleblower law firm can be at obtaining justice. If you are employed in the healthcare industry and have noticed fraudulent misdeeds relating to Medicare or Medicaid, schedule a consultation with our whistleblower lawyers today.
DOJ Resolves More than $1 Billion in False Claims Act Settlements
FCA Settlements and Judgements Exceed $2.9 Billion in Fiscal Year of 2024
EBay to pay $59 Million to Settle Charges Over Pill-Making Machines | Reuters
Glenmark Pharmaceuticals Agrees to Pay $55 Million Settlement | Brown, LLC
Raytheon’s $950 Million Settlement: A Big Win for Whistleblowers and Accountability | Brown, LLC